Background: APL, a rare and aggressive subtype of acute myeloid leukemia (AML), is now curable in 75-90% of patients using targeted agents [All-trans retinoic acid (ATRA)/Arsenic Trioxide (ATO) or ATRA combined with chemotherapy (ATRA+Idarubicin, AIDA-based)]. Despite significant advances in survival, most reports are based on low patient numbers, which limit reliability of data.
Aims: to study factors associated with outcome in a large patient cohort diagnosed with APL, and treated according to two European trials (UK AML-17 and GIMEMA APL0406), or recorded in national registries from 6 countries, enrolled in the HARMONY database.
Methods: The Harmony platform includes to-date 1868 patients with APL, diagnosed between 2007 and 2020. The present analysis was performed on 1296 patients, who met the data quality requirements, by evaluating the list of variables shown in Table 1. Patients were treated according to APL0406 and AML17 clinical trials, or were included in the Study Alliance Leukemia (SAL), Swedish Cooperative Group or AML study Group (AML-SG) registries. After acquisition from the sources, data were harmonized and transformed using an Observational Medical Outcomes Partnership Common Data Model, and registered in the HARMONY Big Data Platform.
Results: Of 1296 patients, 562 were treated with ATRA-ATO (median age 51 yrs, range 16-94; M:F ratio 1), and 605 with ATRA-Idarubicin (AIDA, median age 50 yrs, range 17-86; M:F ratio 1) (p for age groups according to treatment=0.3538). According to Sanz risk-score, 250 patients (44.4%) were low-risk (LR), 258 (45.9%) intermediate-risk (IR), and 50 (8.9%) high-risk (HR) in the ATRA-ATO cohort. The AIDA cohort included 191 LR (31.5%), 235 IR (38.8%), and 171 HR (28.3%) patients (p for risk groups according to treatment: < 0.001). Treatment data were not available in 8 patients (1.3%)
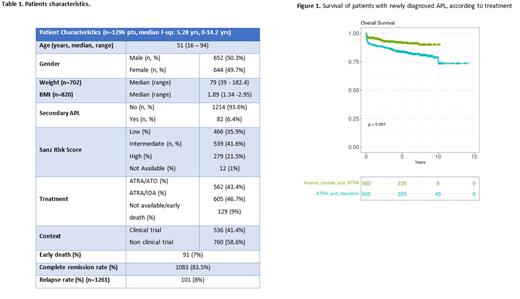
The 10-year overall survival (OS) was 90% and 77% in ATRA-ATO vs AIDA groups, respectively (p<0.001, figure 1), while event-free survival (EFS) was 86% and 67%, respectively (p<0.001).
At a median follow-up of 4.5 yrs (range 0.02 - 10.2), OS and EFS in patients treated with ATRA-ATO was similar in the three Sanz-risk classes (OS: LR: 94.6%, IR: 91%, HR: 85.6%, p=0.233, EFS: LR: 93.1%, IR: 88.7%, HR: 85.7%, p=0.319). The median follow-up of patients treated with AIDA was 6 yrs (range: 0-14.2). OS was 87.8% in LR, 83.5% in IR, and 74.8% in HR (p=0.004), while EFS was 72% in LR, 74.8% in IR, and 67.2% in HR (p=0.102).
OS was significantly associated with age (<50 yrs: n=611, 50-69: n=516, >70: n=169), both overall (p<0.001), and within treatment groups (ATRA-ATO: p<0.001, AIDA: p<0.001). EFS also correlated with age subgroups, both overall (p<0.001) and according to treatment (ATRA-ATO: p<0.001, AIDA: p<0.001). No differences in OS were detected in between de-novo and secondary APL (p=0.746) both in ATRA-ATO (p=0.720) and in AIDA (p=0.766) cohorts.
Patients treated outside the clinical trial context had inferior outcomes when compared to clinical trials, with a significant higher rate of early deaths (ED, 10% vs 2.8%, p<0.001), and inferior survival with major differences for the AIDA cohort (8-year OS: 73.6% vs 88.2%, p<0.001, EFS: 69.3% vs 71.7%, p=0.291), versus the ATRA-ATO cohort (8-year OS: 88% vs 93.3%, p=0.010, 8-years EFS: 80.6% vs 92.4%, p<0.001).
The multivariate analysis for OS showed that age (50-69 vs <50 years, p<0.001, HR 4.2; >70 vs <50 years p<0.001, HR 8.9), Sanz-risk score (High vs Low/Intermediate p<0.001, HR 2), treatment type (AIDA vs ATRA-ATO p=0.001, HR 1.9) and treatment context (clinical trial vs non-clinical trial p=0.005, HR 1.7) were independent predictors of OS.
The multivariate analysis for EFS showed an independent correlation with type of treatment (AIDA vs ATRA-ATO, p<0.001 HR 2.6), age (50-69 vs <50 years, p<0.001, HR 2.1; >70 vs <50 years p<0.001, HR 4.8) and Sanz-risk score (High vs Low/Intermediate p=0.005, HR 1.5), but not with treatment context.
In multivariate analysis treatment type was the only predictive factor for early deaths (AIDA vs ATRA-ATO, p=0.047 HR 2.04).
Summary/Conclusions:
The analysis at long-term of the Harmony APL registry confirmed that patients treated with the ATRA-ATO chemo-free regimen present a significant survival advantage vs the AIDA regimen, with reduced ED rates and prolonged OS, independent of Sanz-risk score. Patients enrolled in clinical trials presented improved survival both in AIDA and in ATRA-ATO cohorts.
Disclosures
Döhner:Abbvie: Consultancy; Daiichi Sankyo: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Astellas: Research Funding; Roche: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; CTI: Consultancy, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Agios: Research Funding. Döhner:Abbvie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Kronos-Bio: Research Funding; Pfizer: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Syndax: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria; Stemline: Consultancy, Honoraria; Berlin-Chemie: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding. Platzbecker:Janssen Biotech: Consultancy, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; Curis: Consultancy, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Geron: Consultancy, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Amgen: Consultancy, Research Funding; Fibrogen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Russell:Servier: Honoraria; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau; Astellas: Honoraria. Dillon:Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AvenCell: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Speakers Bureau; Amgen: Research Funding; Shattuck labs: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Ossenkoppele:Janssen: Consultancy; BMS/Celgene: Consultancy, Honoraria; Pfizer: Research Funding; Astellas: Consultancy, Honoraria; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; JazzPharmaceuticals: Consultancy; Abbvie: Consultancy; Servier: Consultancy; Amgen: Consultancy; Gilead: Consultancy; AGIOS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Vignetti:Novartis: Speakers Bureau; AbbVie: Honoraria; Uvet: Honoraria; Dephaforum: Honoraria; ER Congressi: Honoraria; IQVIA: Honoraria. Bullinger:Daiichi Sankyo: Honoraria; Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Astellas: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Oncology: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hernández-Rivas:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Voso:Abbvie: Speakers Bureau; Celgene/BMS: Other: Advisory Board; Astellas: Speakers Bureau; Astra Zeneca: Speakers Bureau; Novartis: Speakers Bureau; Novartis: Research Funding; Jazz: Other: Advisory Board; Syros: Other: Advisory Board; Jazz: Speakers Bureau; Celgene/BMS: Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal